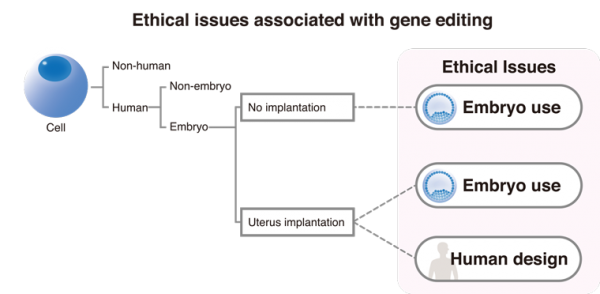
Gene editing the human embryo
In April this year, a research team in China reported the first gene editing of the human embryo. This accomplishment was made in a background of discussion by leading academic journals and societies about the ethics of such editing. Unlike gene editing of other cells, gene editing the embryo potentially changes the entire nature of the body and goes beyond regenerative medicine to human design. Thus, this science addresses bioethical issues that are not relevant to the gene editing of other cell types.
Scientists have long been editing the embryo of non-human animals. Without it, we would not have the many animal disease models that much of our medicine depends. At the same time, there is much research devoted to the gene editing of non-embryonic human cells. Last year, for example, the Hotta lab at CiRA published a study that showed the potential health benefits of gene editing of iPS cells with a mutated dystrophin gene. In the United States, a clinical trial is investigating the effects of cells that underwent gene editing to treat HIV patients.
Gene editing of the embryo brings two special ethical issues to the forefront. The first is the use of the embryo, which has been contentious before gene editing; the second is the potential of human design. These embryos, if placed in the womb, should mature as a natural embryo that emerges upon fertilization. In the aforementioned study, the researchers were clear that the embryo was not and never intended to be returned to the womb, but their standard does not prevent the possibility in future studies by other scientists. At the same time, gene editing of the embryo should provide exceptional opportunities to study the causes of and develop treatment for disease.
This topic easily excites extreme views, with some arguing that no genome editing of the embryo should be permissible and others arguing for much more leniency on its use. Even among scientists, there is wide opinion about how this issue should be handled.
(The first of a two-part series)
by Associate Prof. Misao Fujita, Uehiro Research Division for iPS Cell Ethics
